Research Work
Ubiquitination is a post-translational modification in which one or more ubiquitin molecules are covalently linked to lysine residues of target proteins. The ubiquitin system plays a key role in the regulation of protein degradation, including cell signaling, endocytosis, vesicle trafficking, apoptosis, and immune responses. Bacterial and viral pathogens exploit the cellular ubiquitin system by encoding their own proteins to serve their survival and replication in infected cells. Recent studies have revealed that Kaposi’s sarcoma-associated herpesvirus (KSHV) manipulates cell signaling and the ubiquitin system of infected cells to facilitate cell proliferation, anti-apoptosis, and evasion from immunity.
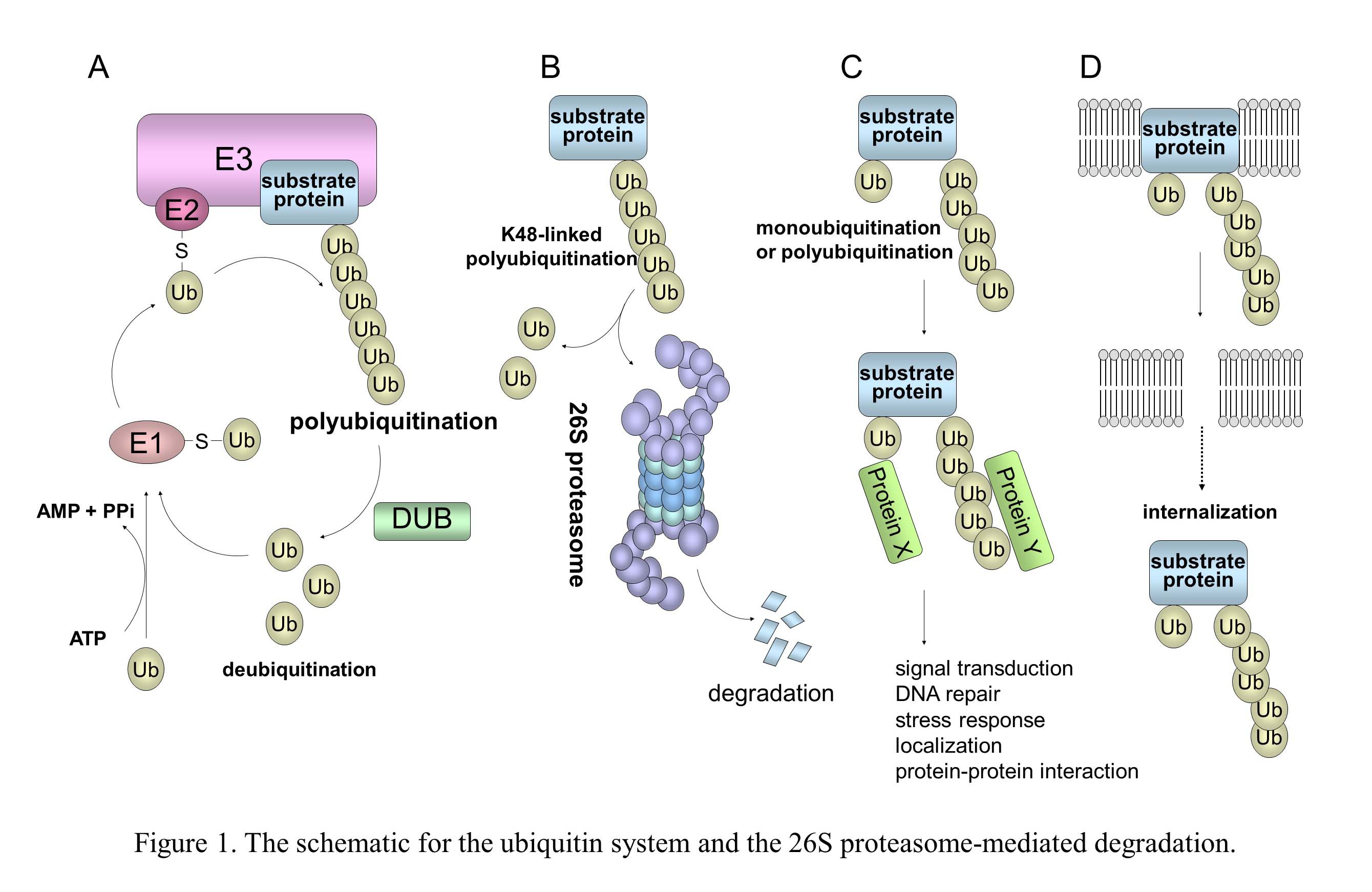
In the ubiquitin system (UPS), a substrate protein is tagged with a polyubiquitin chain or a single ubiquitin molecule via isopeptide bonds that are formed between the carboxyl termini of ubiquitin molecules and either the alpha-amino groups of the lysine residues in the target protein or the ubiquitin molecules themselves (Fig. 1). (A) Ubiquitin conjugation is catalyzed by the sequential actions of ubiquitin-activating enzyme, E1, ubiquitin-conjugating enzyme, E2, and ubiquitin ligase E3. (B) In the case of conjugation with K48-linked polyubiquitin, the polyubiquitinated protein is recognized by the 26S proteasome and is destroyed in an ATP-dependent manner. (C) Modifications of a monoubiquitin and a polyubiquitin chain linked through Lys other than Lys48 (for example, Lys63, Lys11, and Lys29) play a role in many cellular processes, such as signal transduction, DNA repair, and localization through protein-protein interactions. (D) Monoubiquitination and K63-linked polyubiquitination are also involved in the internalization and endocytosis of membrane proteins. It is known that numerous viruses exploit the cellular ubiquitin system in various ways for entry or release of progeny virus and also manipulate this system to abolish cellular defense mechanisms, including apoptosis and the IFN response (Fig. 2). KSHV and HIV-1 utilize the cellular E3 by encoding their own proteins to facilitate cell proliferation, anti-apoptosis, and evasion from immunity.
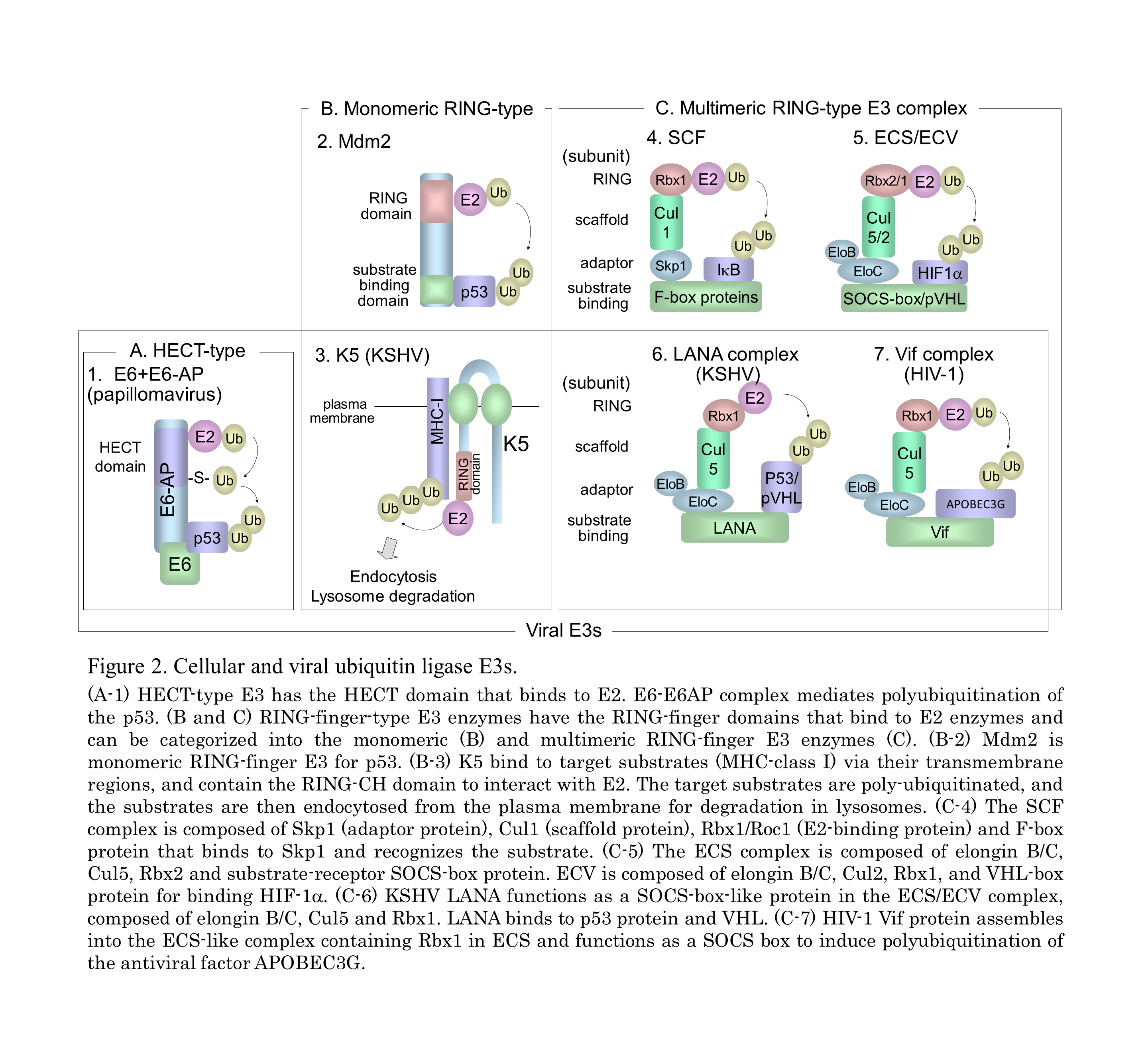
The Kaposi's sarcoma-associated herpesvirus, KSHV was initially identified in Kaposi’s sarcoma (KS), an endothelial neoplasm that is commonly associated with AIDS in homosexual men. It is also associated with primary effusion lymphoma (PEL) and plasmablastic variant Multicentric Castleman’s Disease. KSHV-relating cancers have increased incidence in patients who are also HIV positive or who have undergone organ transplantation. The latency-associated nuclear antigen (LANA), is encoded by ORF73 gene of the KSHV genome, and is a multifunctional protein that ensures the association of viral genome with human genome via a tethering mechanism to facilitate viral DNA replication. LANA also contributes to KSHV-associated oncogenesis through manipulation of pRb and p53 signaling pathway. We have demonstrated that activation of the Wnt signaling through beta-catenin accumulation in KS and PEL. LANA protein stabilizes beta-catenin by binding to the negative regulator GSK-3beta, causing a cell-cycle-dependent nuclear accumulation of GSK-3beta. Thus, KSHV manipulates many cell signaling pathways including Wnt signaling in KSHV-infected cells. Further studies to elucidate KSHV-mediated dis-regulation of the ubiquitin system as well as Wnt signaling and develop novel drug against KSHV infection are currently underway.

Staff and Students
Professor: Masahiro FUJIMURO Ph.D. Researchmap
Lecturer: Yuichi SEKINE Ph.D. Researchmap

